Publications
94. Metal-Catalyzed Divergent Synthetic Methods for Pyrrolocoumarins and Furocoumarins
Maddali L. N. Rao,* Sachchida Nand, Venneti N Murty
Asian J. Org. Chem. 2022, 11, e202100604

93. Pd-Catalyzed cross-coupling synthesis of 4-aryl-3-formylcoumarins
Maddali L. N. Rao,* Sachchida Nand
Org. Bio. Chem. 2022, 20, 1053-1057

Maddali L. N. Rao,* Sk Shamim Islam
Org. Lett. 2021, 23, 8668-8672.

Maddali L. N. Rao,* Sk Shamim Islam
Org. Bio. Chem. 2021, 19, 9076-9080

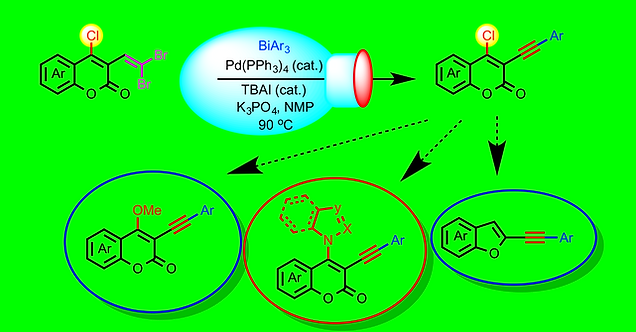
90. Chemo-selective Synthesis of 4-Chloro-3-(arylethynyl)coumarins and their Synthetic Transformations
Maddali L. N. Rao,* Sachchida Nand
Asian J. Org. Chem. 2021, 10, 2944-2949.
89. De Novo Synthesis of Tricyclic 5, 5-Benzannulated Spiroketals
Maddali L. N. Rao,* Sk Shamim Islam
Org. Lett. 2021, 23, 3944–3948

88. Copper-catalyzed domino synthesis of ynamines
Maddali L. N. Rao,* Sk Shamim Islam, Priyabrata Dasgupta
Org. Bio. Chem. 2021, 19, 7855-7860

87. Pd‐Catalyzed Domino Cross‐Coupling: Synthesis of Functionalized 4‐(Arylethynyl) Coumarins
Maddali L. N. Rao,* Sachchida Nand, Venneti N Murty
Asian J. Org. Chem. 2021, 10, 1822-1827

86. Synthesis of alkynes under dry reaction conditions
Maddali L. N. Rao,* Sk Shamim Islam
Tetrahedron Lett. 2021, 71, 153051

85. TS-Torquoselectivity from Global Conformational Profile
Veejendra K Yadav,* Arpita Yadav Maddali L. N. Rao
ChemRxiv. 2021, https://doi.org/10.26434/chemrxiv.13525448.v2
84. On the Thermodynamic Control of Ring Opening of 4-Substituted 1,3,3-Tris-Carbethoxycyclobutene and the Role of
the C-3 Substituent in Masking the Kinetic Torquoselectivity. An alternate reaction pathway
Veejendra Yadav,* Dasari LVK Prasad, Arpita Yadav, Maddali L. N. Rao
ChemRxiv. 2021, https://doi.org/10.26434/chemrxiv.7791146.v5
83. Rapid Bis-coupling Reactivity with Triarylbismuth Reagents: Synthesis of Structurally Diverse Scaffolds and
Step-economic Convergent Synthesis of Quebecol
Maddali L. N. Rao*, Venneti N. Murty, Sachchida Nand
Eur. J. Org. Chem. 2020, 1629-1636

82. Rh-Catalyzed aldehydic C-H alkynylation and annulation
Maddali L. N. Rao,* Boddu S. Ramakrishna
Org. Bio. Chem. 2020, 18, 1402-1411

81. Pd-catalyzed atom-efficient cross-coupling of triarylbismuth reagents with protecting group-free
iodophenylmethanols: Synthesis of biarylmethanols
Maddali L.N. Rao*, Suresh Meka
Tetrahedron Lett. 2020, 61, 151676-151680

80. Pd-catalyzed protecting-group-free cross-couplings of iodophenols with atom-economic triarylbismuth reagents
Maddali L.N. Rao*, Suresh Meka
Tetrahedron Lett. 2020, 61, 151512-151517

79. Rh-Catalyzed Decarbonylative Addition of Salicylaldehydes with Vinyl Ketones: Synthesis of Taccabulins A–E
Maddali L. N. Rao* and Boddu S. Ramakrishna
Eur. J. Org. Chem. 2019, 7545–7554

78. Rh-Catalyzed domino synthesis of 4-hydroxy-3-methylcoumarins via branch-selective hydroacylation
Maddali L. N. Rao, * Boddu S. Ramakrishna and Sachchida Nand
Org. Biomol. Chem. 2019, 17, 9275–9279

77. Atom-efficient Pd-catalyzed cross-couplings of chloroarenes with triarylbismuth reagents
Maddali L.N.Rao, M. Suresh
Tetrahedron Lett. 2019, 60, 150971-150974

76. Rh-Catalyzed Deformylative Coupling of Salicylaldehydes with Acrylates and Acrylamides
Maddali L. N. Rao, B. S. Ramakrishna
J. Org. Chem. 2019, 84, 5677-5683

75. On the Thermodynamic Control of Ring Opening of 4-Substituted 1,3,3-Tris-Carbethoxycyclobutene and the Role
of the C-3 Substituent in Masking the Kinetic Torquoselectivity.
Veejendra Yadav, Dasari LVK Prasad, Arpita Yadav, Maddali L. N. Rao
ChemRxiv. 2019, https://doi.org/10.26434/chemrxiv.7791146.v1
74. On the Conrotatory Ring Opening of 3-Carbomethoxycyclobutene Vis-À-Vis 3-Carbomethoxy-1,2-
Benzocyclobutene and the Predominant Inward Opening of 3-Dimethylaminocarbonyl-1,2-Benzocyclobutene
Veejendra Yadav, Dasari LVK Prasad, Arpita Yadav, Maddali L. N. Rao
ChemRxiv. 2018, https://doi.org/10.26434/chemrxiv.7094003.v1
73. Cross-coupling reactivity of 1,1-dichloroalkenes under palladium catalysis: Domino synthesis of diarylalkynes
Maddali L. N. Rao, M. Suresh
New J. Chem. 2018, 42, 4412-4418

72. Pot-economic synthesis of diarylpyrazoles and pyrimidines involving Pd-catalyzed cross-coupling of
3-trifloxychromone and triarylbismuth
Abhijeet, Kumar, Maddali L. N. Rao
Journal of Chemical Sciences, 2018, 130, UNSP 165

71. Rhodium-Catalyzed Directing-Group-Assisted Aldehydic C–H Arylations with Aryl Halides
Maddali L. N. Rao, B. S. Ramakrishna
Euro. J. Org. Chem. 2017, 5080-5093
70. Substrate-driven selective mono- and bis-couplings of: Ortho -(OTf/I/Br) substituted gem-dibromovinylarenes
Maddali L. N. Rao, P. Dasgupta, S.S. Islam
Org. Chem. Front. 2017, 4, 335-342


69. Functional group manoeuvring for tuning stability and reactivity: Synthesis of cicerfuran, moracins (D, E, M) and
chromene-fused benzofuran-based natural products
Maddali L. N. Rao, V. N. Murty, S. Nand
Org. Bio. Chem. 2017, 15, 9415-9423

68. Rapid regio- and multi-coupling reactivity of 2,3-dibromobenzofurans with atom-economic triarylbismuths
under palladium catalysis
Maddali L. N. Rao, J. B. Talode and V. N. Murty
Beilstein J. Org. Chem. 2016, 2065-2076

67. Rh-catalyzed direct synthesis of 2,2′-dihydroxybenzophenones and xanthones
Maddali L. N. Rao, B. S. Ramakrishna
RSC Advances 2016, 6, 75505-75511

66. Rapid Access to Benzofuran-Based Natural Products through a Concise Synthetic Strategy
Maddali L. N. Rao* and Venneti N. Murty
Eur. J. Org. Chem. 2016, 6, 2177-2186

65. Rapid threefold cross-couplings with sterically bulky triarylbismuths under Pd-Cu dual catalysis
Maddali L. N. Rao,* and Ritesh J. Dhanorkar
RSC Adv. 2016, 6, 1012-1017

64. Rapid access to triarylated cyclotriveratrylenes from threefold couplings of CTV-I3 with triarylbismuth reagents
Maddali L. N. Rao*, J. B. Talode
Asian J. Org. Chem. 2016, 5, 98-106

63. Rapid access to unsymmetrical 1,3-diynes and 2,5-disubstituted thiophenes under ligand and Pd/Ni free
Cu-catalysis
Maddali L. N. Rao,* Sk S. Islam, P. Dasgupta
RSC Adv. 2015, 5, 78090-78098

62. A concise route to functionalized benzofurans directly from gem-dibromoalkenes and phenols
Maddali L. N. Rao*, P. Dasgupta
RSC Adv. 2015, 5, 65462-65470


61. Pd-catalyzed cross-coupling study of bi-functional 3-bromo-4-trifloxycoumarins with triarylbismuth reagents
Maddali L. N. Rao*, A. Kumar
Tetrahedron 2015, 71, 5137-5147


60. De novo synthesis of functionalized 1,3-eneyne and extended conjugated molecular systems
Maddali L. N. Rao*, P. Dasgupta, V. N. Murty
RSC Adv. 2015, 5, 24834-24845

59. Cross-coupling study of iodo/chloropyridines and 2-chloroquinoline with atom-economic triarylbismuth reagents
under Pd-catalysis
Maddali L. N. Rao*, R. J. Dhanorkar
Tetrahedron 2015, 71, 338-349


58. Pd-catalyzed atom-economic couplings of triarylbismuth reagents with 2-bromo- and 2,6-dibromochromones
and synthesis of medicinally important fisetin
Maddali L.N. Rao*, A. Kumar
Tetrahedron Lett. 2014, 55, 5764-5770

57. Atom-economic threefold cross-coupling of triarylbismuth reagents with 2- halobenzaldehydes and
pot-economic in situ Wittig functionalization with phosphonium salts
Maddali L.N. Rao*, R. J. Dhanorkar
RSC Adv. 2014, 4, 63792-63806

56. Combined catalysis: Pd-catalyzed two-step one-pot protocol for 2,3-diaryl-1-indenones involving domino
synthesis of diarylacetylenes and Heck–Larock annulations
Maddali L. N. Rao*, R. J. Dhanorkar
Tetrahedron 2014, 70, 8067-8078

55. Pd-catalyzed chemo-selective mono-arylations and bis-arylations of functionalized 4-chlorocoumarins with
triarylbismuths as threefold arylating reagents
Maddali L. N. Rao*, A. Kumar
Tetrahedron 2014, 70, 6995-7005

54. Triarylbismuthanes as threefold aryl-transfer reagents in regioselective cross-coupling reactions with
bromopyridines and quinolines
Maddali L. N. Rao*, R. J. Dhanorkar
Eur. J. Org. Chem. 2014, 5214-5228



53. Domino synthesis of 1,3-diynes from 1,1-dibromoalkenes: A Pd-catalyzed copper-free coupling method
Maddali L. N. Rao*, P. Dasgupta, B. S. Ramakrishna, V. N. Murty
Tetrahedron Lett. 2014, 55, 3529-3533

52. Threefold and chemoselective couplings of triarylbismuths with benzylic chlorides and iodides using
palladium catalysis
Maddali L. N. Rao*, R. J. Dhanorkar
RSC Adv. 2014, 4, 13134-13144



51. Pd-catalyzed chemoselective threefold cross-coupling of triarylbismuths with benzylic bromides
Maddali L. N. Rao*, R. J. Dhanorkar
RSC Adv. 2013, 3, 6794-6798

50. Pd-catalyzed tandem chemoselective synthesis of 2-arylbenzofurans using threefold arylating triarylbismuth
reagents
Maddali L. N. Rao*, D. N. Jadhav, P. Dasgupta
Eur. J. Org. Chem. 2013, 781-788

49 Pd-catalyzed threefold arylations of mono, di and tetra-bromoquinones using triarylbismuth reagents
Maddali L. N. Rao*, S. Giri
RSC Adv. 2012, 2, 12739-12750


48. Triphenylbismuthane (Invited)
Maddali L. N. Rao
Encyclopedia of Reagents for Organic Synthesis John & Wiley, 2012

47. Pd-catalyzed threefold arylation of Baylis–Hillman bromides and acetates with triarylbismuth reagents
Maddali L. N. Rao*, S. Giri
Eur. J. Org. Chem. 2012, 4580-4589

46. Mono- and biscouplings using triarylbismuths for the atom-efficient arylations of functionalized furans under
palladium catalysis
Maddali L. N. Rao*, D. K. Awasthi, J. B. Talode
Synlett 2012, 23, 1907-1912

45. Palladium-catalyzed cross-couplings of functionalized 2-bromobenzofurans for atom-economic synthesis of
2-arylbenzofurans using triarylbismuth reagents
Maddali L. N. Rao*, D. K. Awasthi, J. B. Talode
Tetrahedron Lett. 2012, 53, 2662-2666

44. Transition-metal catalyzed C-C bond formation using organobismuth compounds
S. Shimada, Maddali L. N. Rao
Topics in Current Chemistry 2012, 311, 199-228

43. Palladium catalyzed atom-economic synthesis of functionalized 9-(diarylmethylene)-9H-fluorenes using
triarylbismuths in one-pot bis-coupling process
Maddali L. N. Rao*, P. Dasgupta
Tetrahedron Lett. 2012, 53, 162-165

42. Synthesis of functionalized 2-arylthiophenes with triarylbismuths as atom-efficient multi-coupling
organometallic nucleophiles under palladium catalysis
Maddali L.N. Rao*, D. Banerjee, R. J. Dhanorkar
Synlett 2011, 1324-1330


41. Palladium-catalzed novel arylations of cyclic β-bromo-α, β-unsaturated aldehydes with triarylbismuths as multi-
coupling organometallic nucleophiles
Maddali L.N. Rao*, D. Banerjee, R. J. Dhanorkar
Synlett 2011, 273-279
40. Pd-catalyzed couplings of aryl iodides with triarylbimsuths as atom-economic multi-coupling orgnaometallic
nucleophiles under mild conditions
Maddali L.N. Rao*, D. Banerjee, R. J. Dhanorkar
Tetrahedron Lett. 2010, 51, 6101-6104

39. Oxalyl chloride as carbonyl synthon in Pd-catalyzed carbonylations of triarylbismuth and triarylindium
organometallic nucleophiles
Maddali L. N. Rao*, V. Venkatesh, P. Dasgupta
Tetrahedron Lett. 2010, 51, 4975-4980

38. Palladium-catalyzed synthesis of 4-arylcoumarins using triarylbismuth compounds as atom-efficient multi-
coupling organometallic nucleophiles
Maddali L. N. Rao*, V. Venkatesh, D. N. Jadhav
Eur. J. Org. Chem. 2010, 3945-3955


37. Pd(0)-catalyzed couplings using bromide and chloride derivatives of Baylis–Hillman adducts with triarylbismuths
as atom-efficient multi-coupling nucleophiles
Maddali L. N. Rao*, D. Banerjee, R. J. Dhanorkar
Tetrahedron 2010, 66, 3623-3632
36. Palladium catalyzed cross-couplings of allylic carbonates with triarylbismuths as multi-coupling atom-efficient
organometallic nucleophiles
Maddali L. N. Rao*, D. Banerjee, S. Giri
J. Organomet. Chem. 2010, 695, 1518-1525

35. Pd-catalyzed domino synthesis of internal alkynes using triarylbismuths as multicoupling organometallic
nucleophiles
Maddali L. N. Rao*, D. N. Jadhav, P. Dasgupta
Org. Lett. 2010, 12, 2048-2051

34. An expeditious and convergent synthesis of ailanthoidol
Maddali L. N. Rao*, D. K. Awasthi, D. Banerjee
Tetrahedron Lett. 2010, 51, 1979-1981


33. Pd-catalyzed synthesis of α-aryl ketones through couplings of α-arylacetyl chlorides with triarylbismuths as
multi-coupling nucleophiles
Maddali L. N. Rao*, S. Giri, D. N. Jadhav
Tetrahedron Lett. 2009, 50, 6133-6138
32. Pd-catalyzed efficient cross-couplings of 3-iodochromones with triarylbismuths as sub-stoichiometric
multicoupling organometallic nucleophiles
Maddali L. N. Rao*, V. Venkatesh, D. N. Jadhav
Synlett 2009, 2597-2600

31. Palladium catalyzed allylic acetates using triarylbismuths as sub-stoichiometric atom-efficient multi-coupling
reagents under palladium catalysis
Maddali L. N. Rao*, D. Banerjee, S. Giri
Tetrahedron Lett. 2009, 50, 5757-5761

30. Atom-efficient vinylic arylations with triarylbismuths as substoichiometric multi-coupling reagents under
palladium catalysis
Maddali L. N. Rao*, D. N. Jadhav,V. Venkatesh
Eur. J. Org. Chem. 2009, 4300-4306

29. Pd(0)/C catalyzed cross-couplings of acyl chlorides with triarylbismuths as atom-efficient sub-stoichiometric
multi-coupling reagents
Maddali L. N. Rao*, D. N. Jadhav, V. Venkatesh
Tetrahedron Lett. 2009, 50, 4268-4271

28. A palladium catalyzed atom-efficient cross-coupling reactivity of triarylbismuths with α,β-unsaturated acyl
chlorides
Maddali L.N. Rao*, V. Venkatesh, D. N. Jadhav
J. Organomet. Chem. 2008, 693, 2494-2498

27. A new palladium catalyzed protocol for atom-efficient cross-coupling reactions of triarylbismuths with aryl
halides and triflates
Maddali L.N. Rao*, D. N. Jadhav, D. Banerjee
Tetrahedron 2008, 64, 5762-5772



26. Atom-efficient cross-coupling reactions of triarylbismuths with acyl chlorides under Pd(0) catalysis
Maddali L. N. Rao*, V. Venkatesh, D. Banerjee
Tetrahedron 2007, 63, 12917-12926

25. Palladium catalyzed atom-efficient cross-coupling reactions of triarylbismuths with aryl iodides and aryl
triflates
Maddali L. N. Rao*, D. Banerjee, D. N. Jadhav
Tetrahedron Lett. 2007, 48, 6644-6647

24. Palladium catalyzed atom-efficient cross-coupling reactions of triarylbismuths with aryl bromides
Maddali L. N. Rao*, D. Banerjee, D. N. Jadhav
Tetrahedron Lett. 2007, 48, 2707-2711

23. Microwave-mediated solvent free Rap-Stoermer reaction for efficient synthesis of benzofurans
Maddali L. N. Rao*, D. K. Awasthi, D. Banerjee
Tetrahedron Lett. 2007, 48, 431-434

22. An atom-efficient palladium-catalyzed cross-coupling reaction of triarylbismuths with acid chlorides:
Synthesis of diaryl and alkyl aryl ketones
Maddali L. N. Rao*, V. Venkatesh, D. N. Jadhav
Tetrahedron Lett. 2006, 47, 6975-6978

21. Metal catalyst-free direct α-iodination of ketones with molecular iodine
Maddali L.N. Rao*, D. N. Jadhav
Tetrahedron Lett. 2006, 47, 6883-6886

20. A Pt(III)2Si2 four-membered cycle and a dinuclear platinum complex bridged by a cyclodisiloxane ring
S. Shimada, Y.-H. Li, Maddali L. N. Rao, M. Tanaka*
Organometallics 2006, 25, 3796-3798

19. Reaction of 1-(Dimethylsilyl)-2-silylbenzene with platinum(0) phosphine complexes
S. Shimada, Maddali L. N. Rao, Y.-H. Li, M. Tanaka*
Organometallics 2005, 24, 6029-6036

18. 5,6,7,12-Tetrahydrobenz[c,f][1,5]azabismocines: Highly reactive and recoverable organobismuth reagents for cross-
coupling reactions with aryl bromides
S. Shimada, O. Yamazaki, T. Tanaka, Maddali L. N. Rao, Y. Suzuki, M. Tanaka*
Angew. Chem. Int. Ed. 2003, 42, 1845-1848

17. Cross-coupling reaction of organobismuth dialkoxides with aryl bromides and iodides catalyzed by Pd(PPh3)4
Maddali L. N. Rao, S. Shimada, O. Yamazaki, M. Tanaka*
J. Organomet. Chem. 2002, 659, 117-120

16. Self-assembly of double stranded novel titanium(IV)-Schiff base complexes and the formation of intramolecular
m-oxo bridges
Maddali L. N. Rao, H. Houjou, K. Hiratani*
Chem. Commun. 2002, 420-421

15. Novel synthesis of macrocycles with chalcone moieties through mixed aldol reaction
Maddali L. N. Rao, H. Houjou, K. Hiratani*
Tetrahedron Lett. 2001, 42, 8351-8355

14. Palladium catalyzed cross-coupling reactions of triarylbismuths with aryl halides and triflates
Maddali L. N. Rao, O. Yamazaki, S. Shimada, T. TanakaY. Suzuki and M. Tanaka*
Org. Lett. 2001, 3, 4103-4105

13. Isolation of a dinuclear(m-Silylene)(silyl)nickel complexes and Si-Si bond formation on a dinuclear nickel
framework
S. Shimada, Maddali L. N. Rao , T. Hayashi, M. Tanaka*
Angew. Chem. Int. Ed. 2001, 40, 213-216

12. Synthesis and structure of monoorganobismuth compounds bearing pyridine dimethoxide ligands
S. Shimada, Maddali L. N. Rao, M. Tanaka*
Organometallics 2000, 19, 931-936

11. Reaction of 1,2-disilylbenzene with bis(1,2-bis(dimethylphosphino) ethane)- nickel(0): isolation and
characterization of the first silylnickel(IV) complex
S. Shimada, Maddali L. N. Rao, M. Tanaka*
Organometallics 1999, 18, 291-293

10. Palladium complex-catalyzed cross-coupling reaction of organobismuth- dialkoxides with triflates
Maddali L. N. Rao, S. Shimada, M. Tanaka*
Org. Lett. 1999, 1, 1271-1273

9. New reactivity of (RC2CH2OH)Co2(CO)6 complexes in Pauson-Khand reaction in the presence of CF3COOH:
Synthesis of methyl substituted cyclopentenones
M. Periasamy*, Madddali L. N. Rao, T. Rajesh
J. Organomet. Chem. 1998, 571, 183-187

8. The CoCl2/Ph(Et)2N:BH3/CO system: reactions of the borane and cobalt carbonyl species
Maddali L. N. Rao, M. Periasamy*
J. Organomet. Chem. 1998, 553, 91-97

7. Reductive Pauson-Khand reaction using (RC2R')Co2(CO)6/CF3COOH system
Maddali L. N. Rao, M. Periasamy*
J. Organomet. Chem. 1997, 532, 143-146

6. Novel syntheses of cyclopentenones and alkenylsilanes from the corresponding alkyne-dicobalt hexacarbonyl
complexes
Maddali L. N. Rao, M. Periasamy*
Organometallics 1996, 15, 442-445

5. Synthesis of dialkyl ketones from alkenes using Ph(Et)2N:BH3/CoCl2 /CO reagent system
Maddali L. N. Rao, M. Periasamy*
Tetrahedron Lett. 1995, 36, 9069-9070

4. Cobalt(II) acetate as reagent for organic synthesis
M. Periasamy*, Maddali L. N. Rao
Encyclopedia of Reagents for Organic Synthesis
John & Wiley,1995, 2, 1284-1286

3. Cobalt(II)chloride as reagent for organic synthesis
M. Periasamy*, Maddali L. N. Rao
Encyclopedia of Reagents for Organic Synthesis
John & Wiley, 1995, 2, 1291-1293

2. A simple convenient method for the synthesis of 1-iodoalkynes
Maddali L. N. Rao, M. Periasamy*
Synth. Commun. 1995, 25, 2295-2299

1. Carbonylation of R2BI in the presence of NaCo(CO)4 and Na2Fe(CO)4: Simple synthesis of dialkyl ketones
A. Devasagayaraj, Maddali L. N. Rao, M. Periasamy*
J. Organomet. Chem. 1991, 421, 147-150

Triarylbismuths as Threefold Organometallic Reagents in Organic Synthesis



